By Tyrone Burke
Photos by Chris Roussakis
Deep in the Pacific, against a backdrop of liquid darkness, a virtual armada of jumbo squid lay in wait. More than 1,000 strong, they bob in a state of suspended animation hundreds of metres below the surface. Once night sets in, more than 1,000 will ascend to the surface for a feeding frenzy and ravenously devour their prey.
And the prey? That can even include humans if they’re in the wrong place at the wrong time.
A school of jumbo squid is scarier than a Sharknado from those cult movies and it’s mostly because they’re an actual thing. In Mexico, fishermen nicknamed them “diablo rojo” – the red devil. They’ve attacked submarines and scuba divers. Fishermen who are unfortunate enough to fall into squid-infested waters sometimes never emerge.

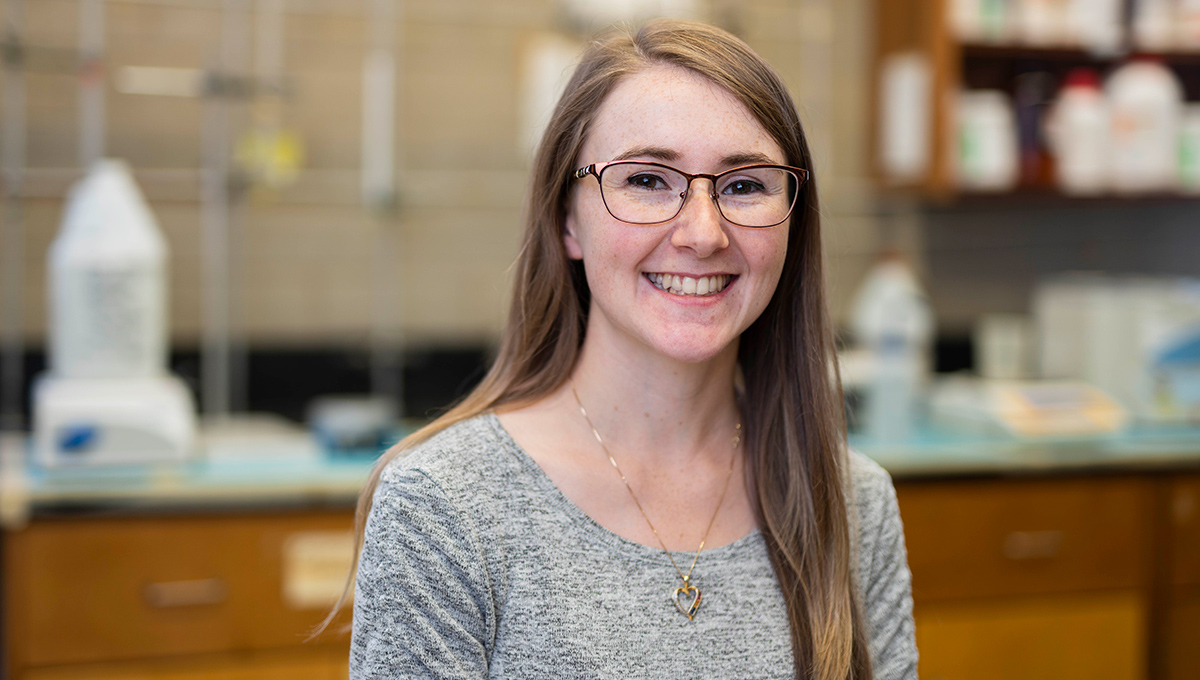
Hanane Hadj-Moussa
Jumbo squid migrate between the surface and a depth of about 300 metres – where the pressure is much higher and the oxygen and water temperatures are far lower. Carleton University’s Hanane Hadj-Moussa wanted to understand how they were doing it.
“Every day, jumbo squid descend to the ocean’s oxygen minimum zone,” says Hadj-Moussa.
“There, they depress their metabolism by up to 50 per cent. They can just hang out there while they digest their food and avoid predators. When the sun goes down, they go back up. We were interested in how they survive the extreme pressure, frigid temperatures and severe hypoxic conditions (low oxygen) and how they reverse it — and do that every day.”

Identifying Jumbo Squid MicroRNA
A PhD student in biology who studies cell and molecular responses to stress in Prof. Ken Storey’s lab, Hadj-Moussa worked with fellow PhD student Samantha Logan to identify jumbo squid microRNA in research funded through experiment.com, a crowdfunding platform for scientific experiments.
MicroRNA molecules repress the function of an animal’s genes. They have reversible effects and can target almost any aspect of biological function. Hibernating animals use microRNA to ensure that bodily functions are preserved – keeping organs like the heart and brain in working order during prolonged periods of inactivity.
Hadj-Moussa and Logan identified 39 types of microRNA that jumbo squid could use to repress most of their bodily functions while in the extreme deep water environment, then reactivate their bodies when they resurface to hunt.
Samantha Logan
“We looked at how squid regulate microRNA at normal oxygen levels, versus simulated hypoxic – low oxygen — conditions like those found at the bottom of the ocean,” says Hadj-Moussa
“What we found was that there are specific microRNAs that reprogram the squid’s metabolism, while others have neuroprotective functions or act to inhibit programmed cell death. The squid use microRNA to overcome a lot of challenges that other animals would not be able to overcome and survive in extreme conditions.”
Yet even in those conditions, the jumbo squid’s metabolism is still half its normal rate. Other animals are able to use microRNA to make their vital signs virtually disappear.
“In our lab, we look at microRNA responses across all sorts of animal survival strategies and metabolic rate depression strategies,” says Hadj-Moussa, who values the opportunity to do novel work that comes with doing her PhD in Storey’s lab.
“Some frogs can freeze up to 70 per cent of their bodies. They have no heart rate, no brain activity, no moving, no breathing. They can do this for months. When we look at their brain, even though there’s no measurable brain activity, the microRNA responses suggest that various neuroprotective functions are being turned on, even though we don’t see electrical conductance, we do see neural networks being maintained and axons being preserved so that the frog maintains those functions.”
Zombie Gene Behaviour Likened to Extreme Survival Strategies in Jumbo Squids
Hadj-Moussa wondered if genes in animals with extreme survival strategies behaved in similar ways to animals that have actually died. Some genes actually become more active in the days after death, exhibiting what Hadj-Moussa calls a zombie gene expression profile.
Many of the genes that become more active after death are involved in survival, such as those involved in anti-apoptosis (programmed cell death) and heat shock. And these are the types of genes that are often active in metabolic rate suppression in animals.
“Animals that live in these extreme conditions are the closest living things to death, if that makes sense,” says Hadj-Moussa, who conducted the zombie gene research with PhD student Alex Watts.

Alex Watts
“I was curious whether animals that undergo hibernation, freeze tolerance or anoxia tolerance would express the same zombie gene expression profile.”
As it turns out, they do not. That could be because animals’ extreme survival strategies are controlled, while control functions are lost in the process of dying. On a molecular level, death is a gradual process that leaves some cells grasping at life, even as others have already died.
“Not all or your cells are instantaneously dead,” says Hadj-Moussa.
“Death is a progression. As you die, the cells on the inside starve of oxygen first. The zombie genes we are seeing are genes involved in the cell’s last resort to overcoming stress before unequivocal death.”

Monday, October 28, 2019 in Faculty of Science, Research
Share: Twitter, Facebook



